Initial Positive Efficacy Data From SAVVE Trial
11-Month Topline Revised Venous Clinical Severity Score (rVCSS) Improvement*
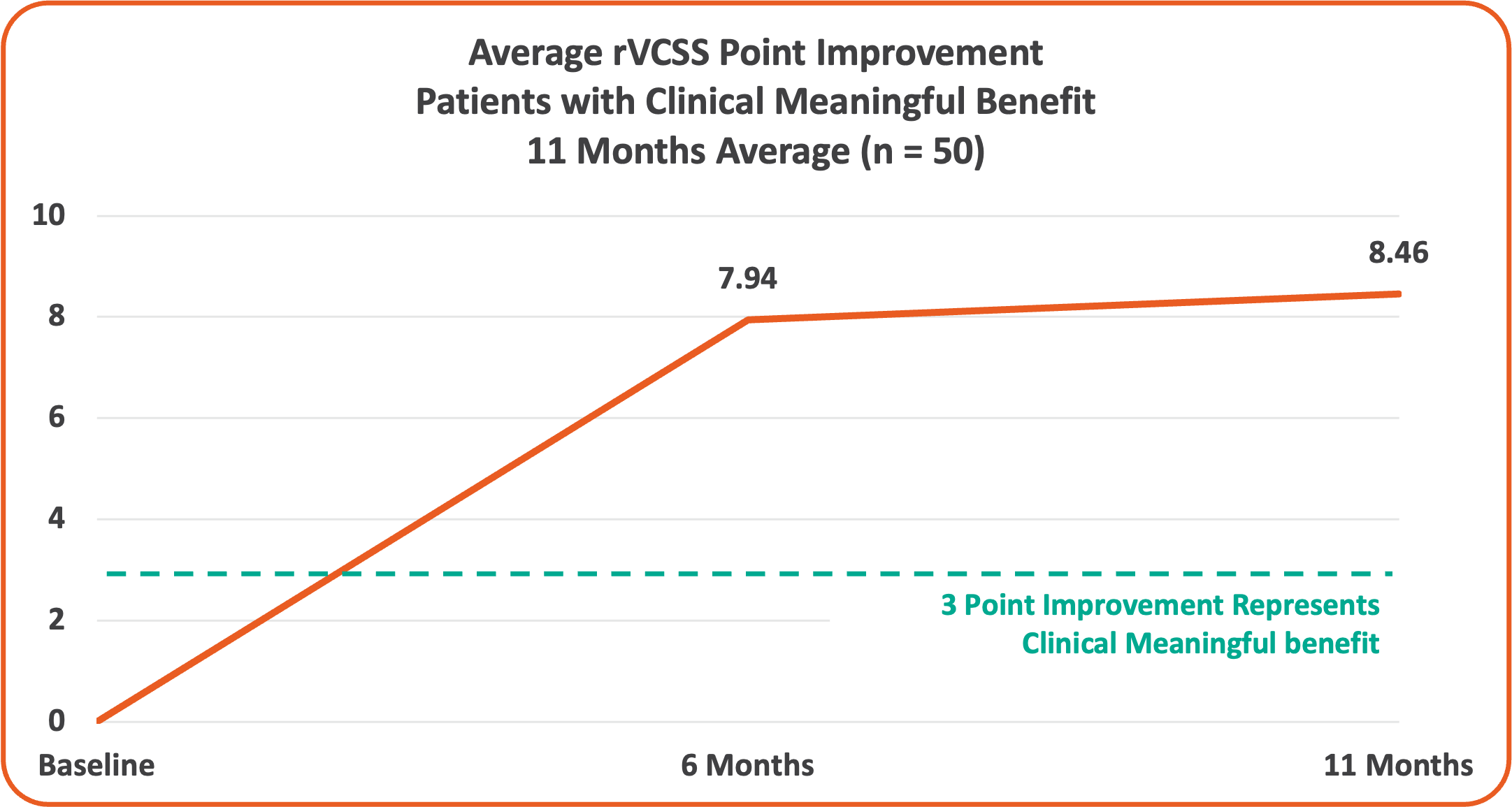
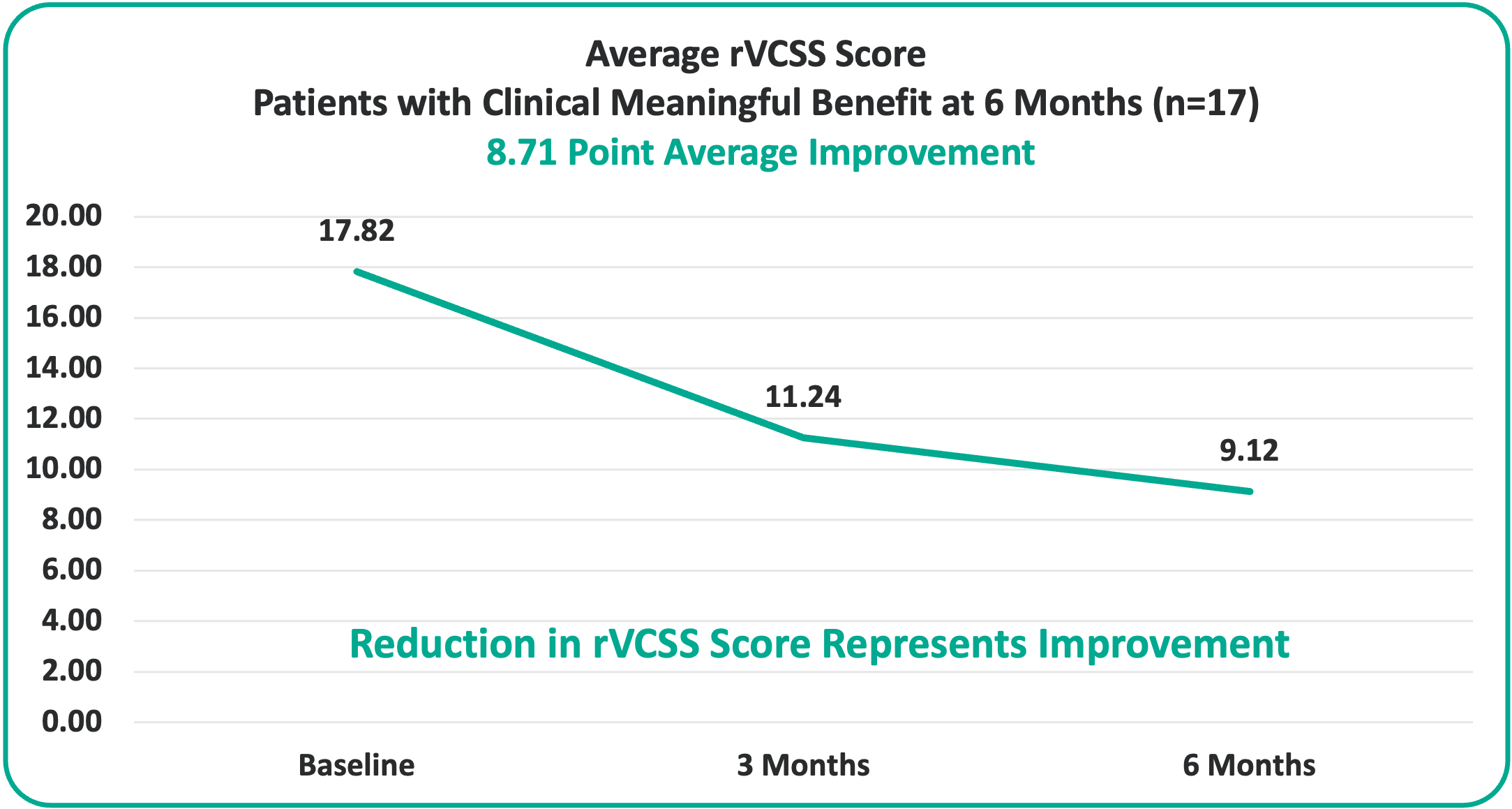
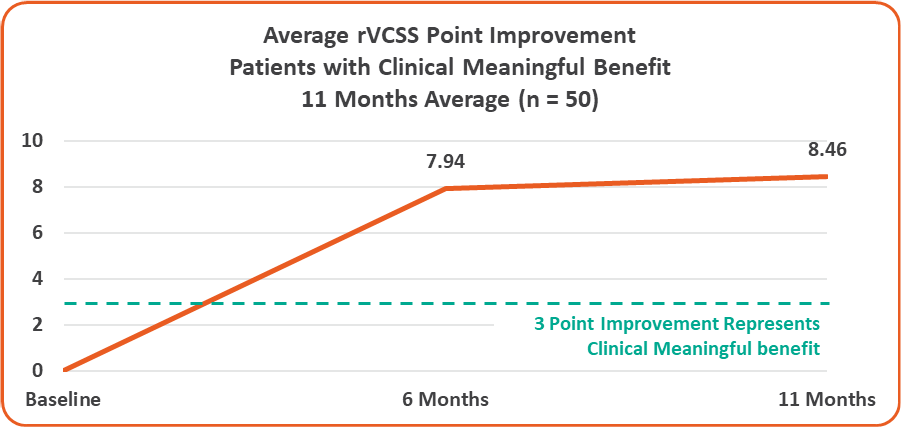
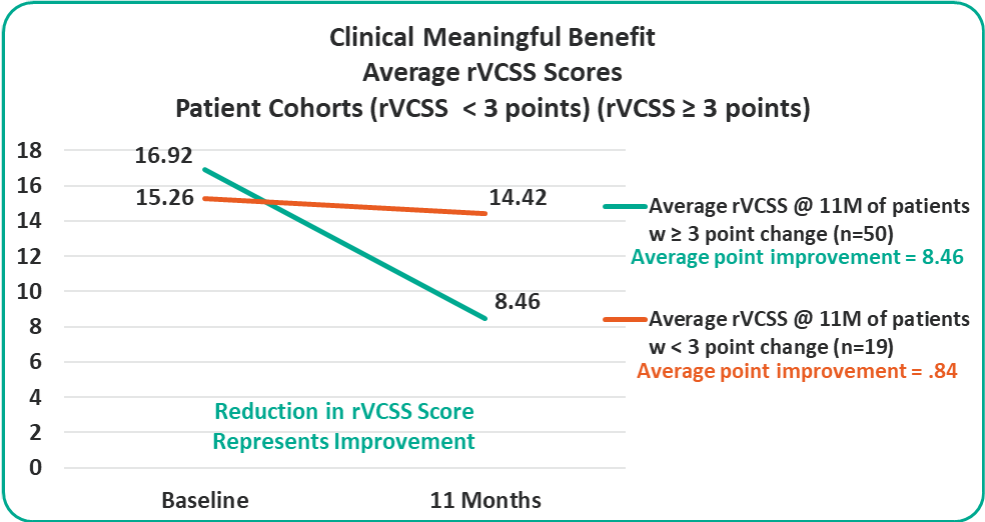
• Overall 8.46 Average Revised Venous Clinical Severity Score (rVCSS) Improvement Per Patient for Patients Showing Clinical Meaningful Benefit (rVCSS Improvement ≥ 3 Points) including:
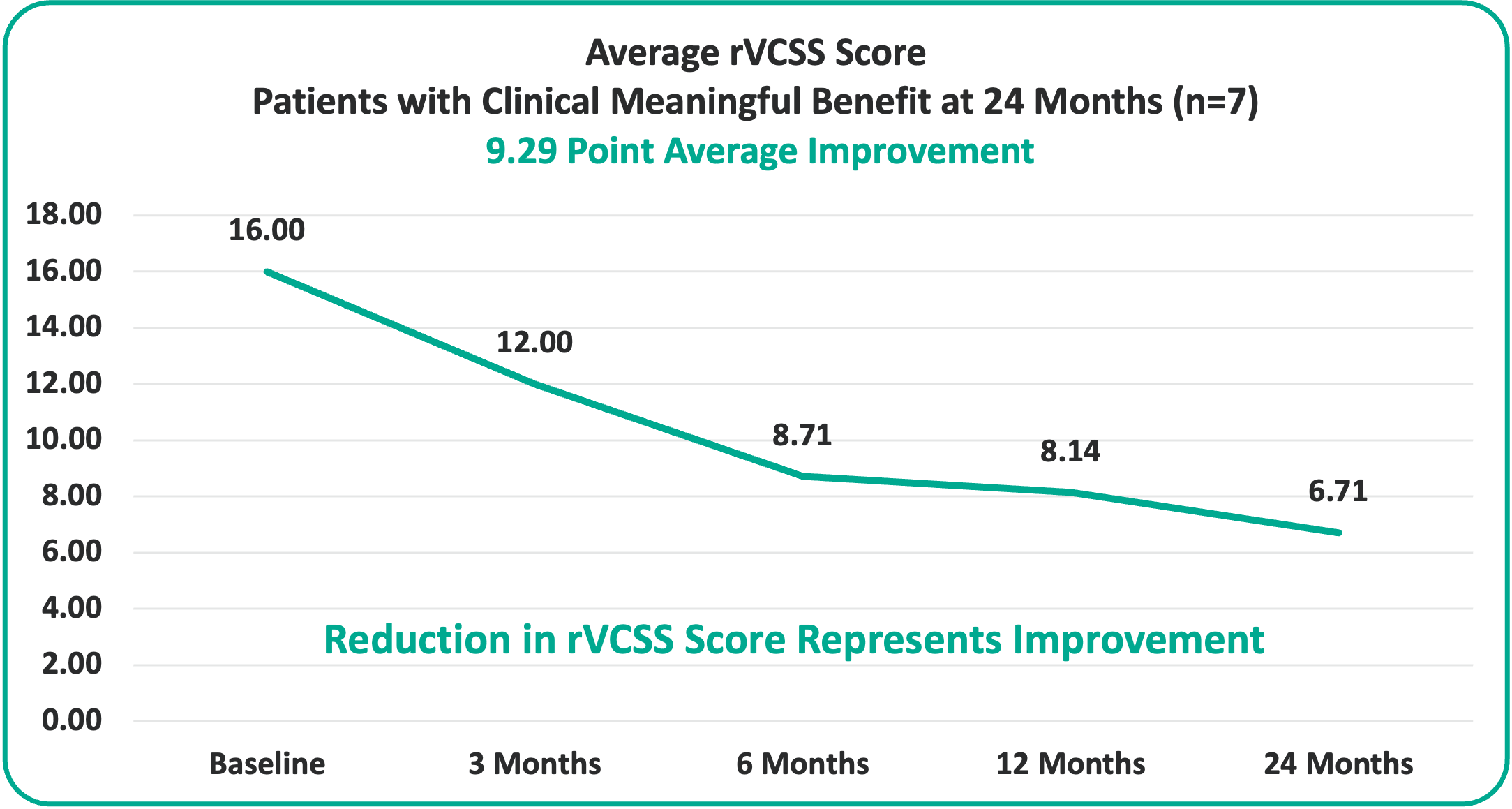
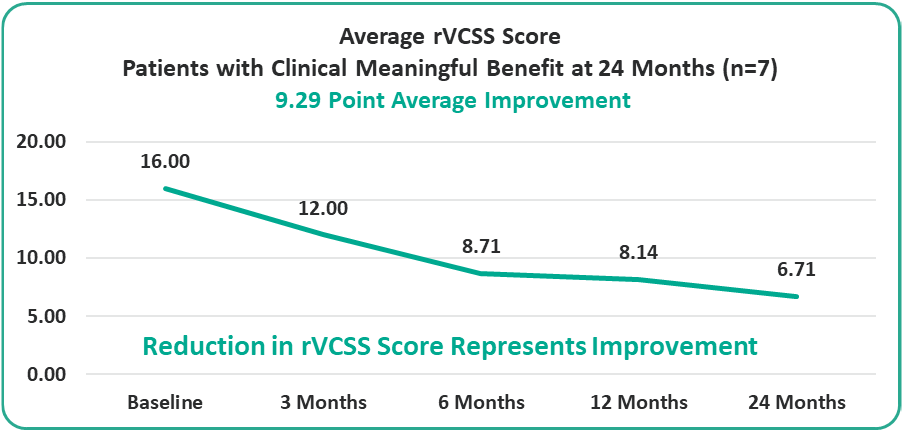
• 9.29 Points for Patients at the Two-Year Milestone
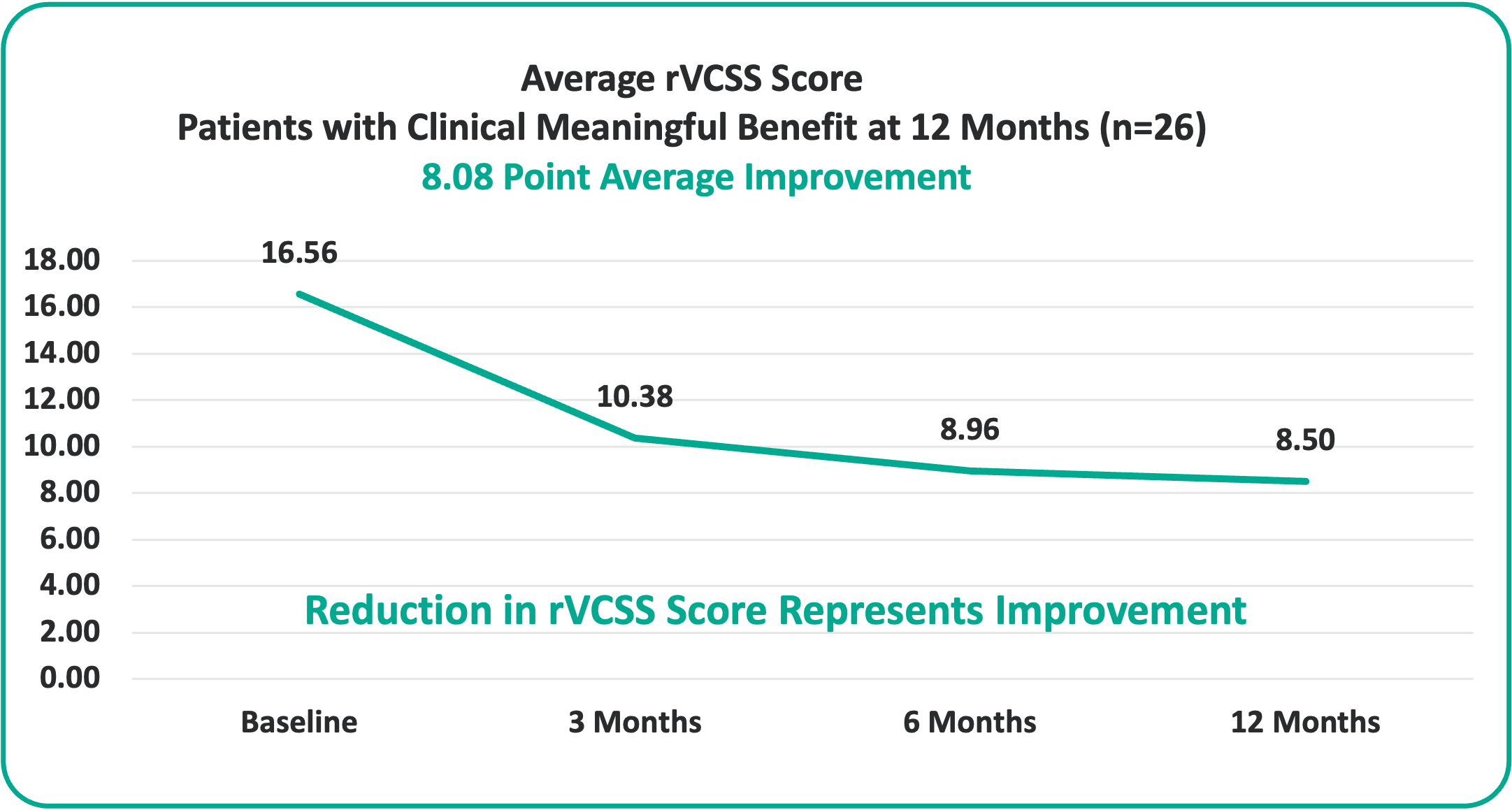
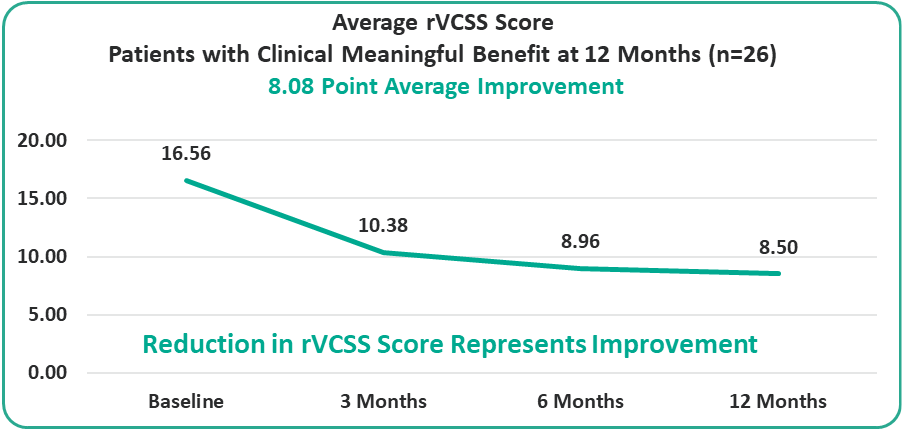
• 8.08 Points for Patients at the One-Year Milestone
• 8.71 Points for Patients at the Six-Month Milestone
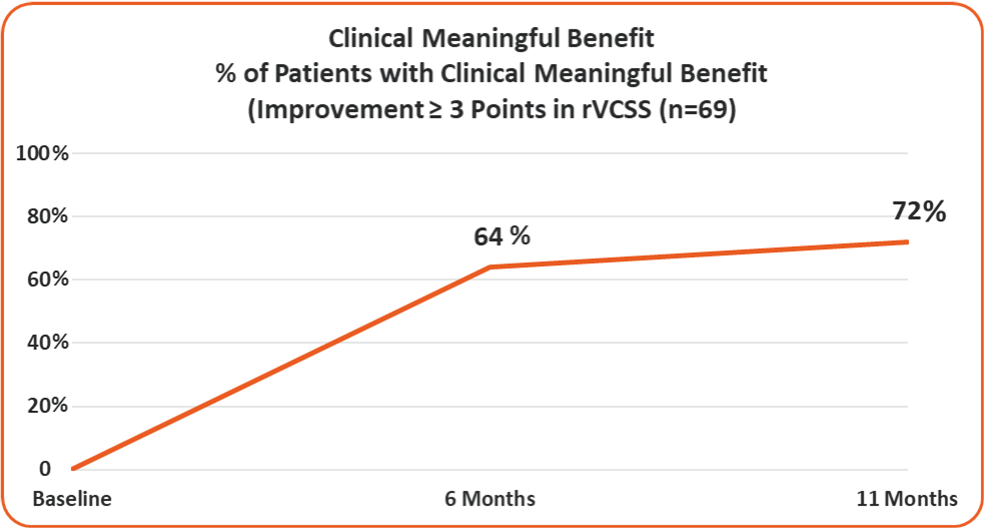
• 72% of the Study Patients Showing Clinical Meaningful Benefit from the VenoValve at a Weighted Average of 11 Months Post Surgery
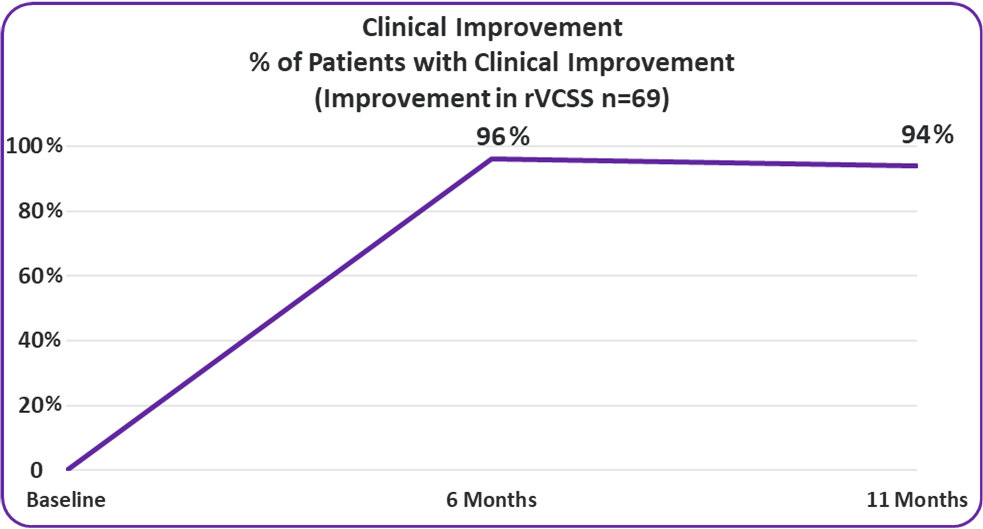
• 94% of VenoValve Study Patients Showing Clinical Improvement at a Weighted Average of Eleven Months Post Surgery (rVCSS Improvement ≥ 1 point)
* Data compared to baseline
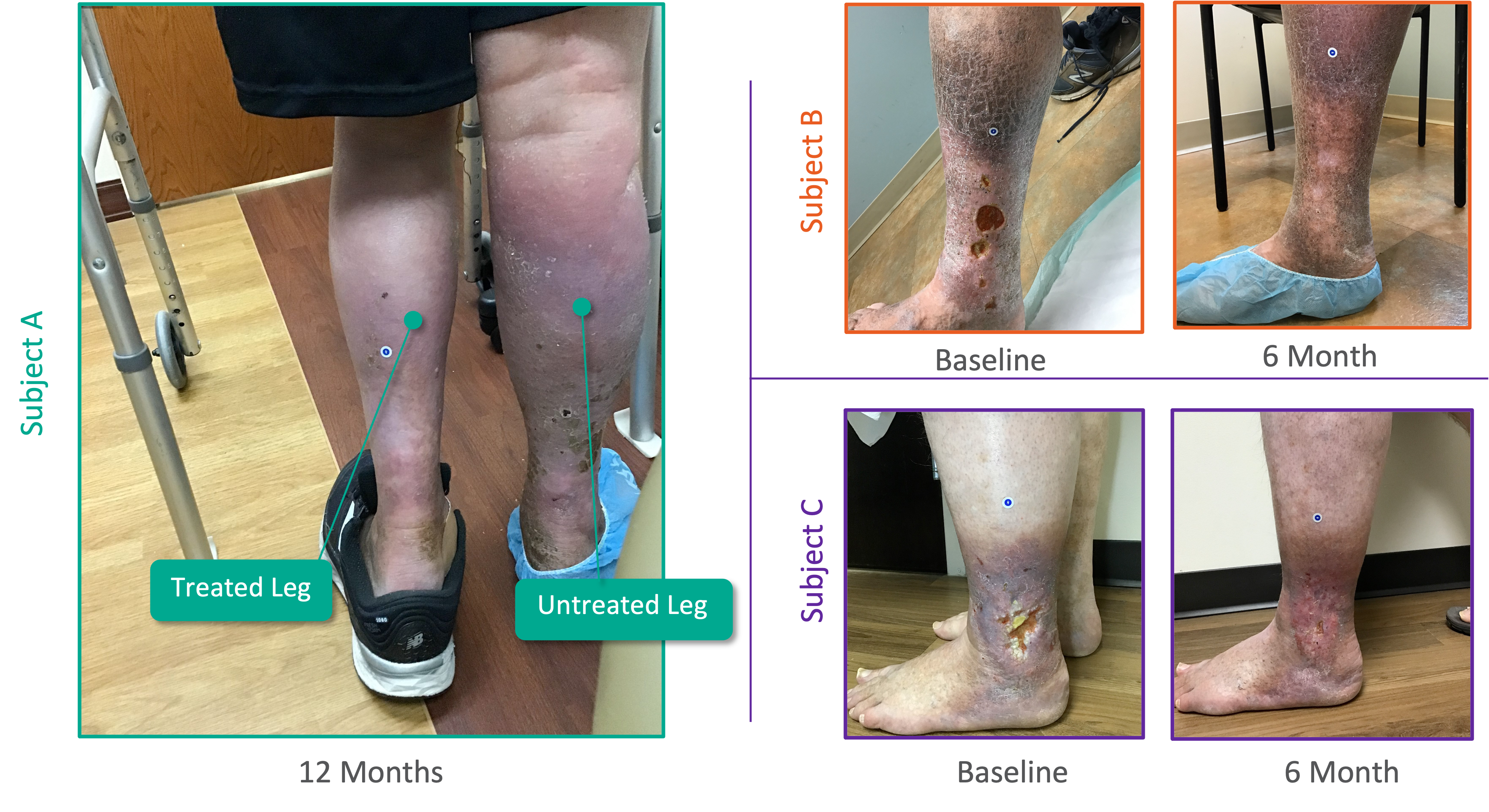
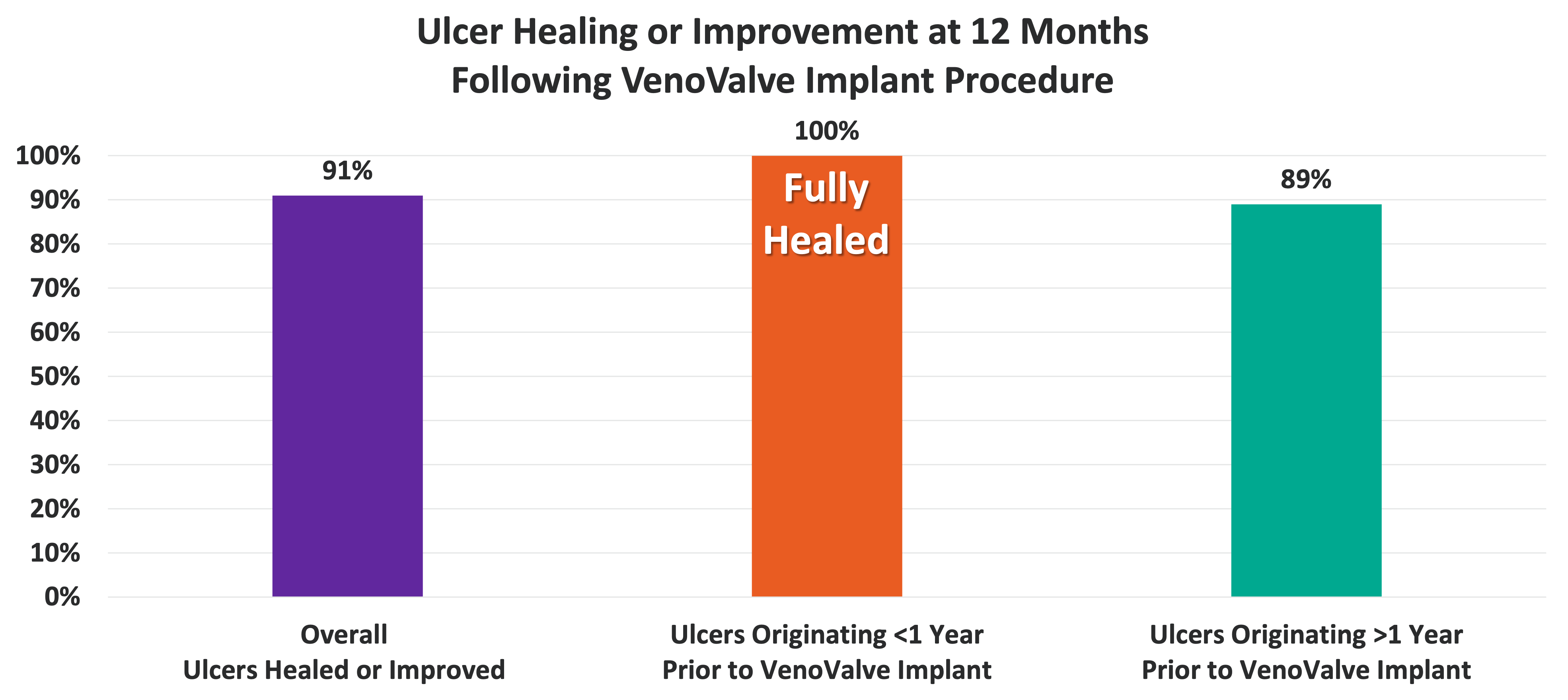
12 Month Ulcer Healing Data Showing Significant Improvement
• 91% of Venous Ulcer Patients Evaluated at One Year Have Either Fully Healed Ulcers or Ulcers That Have Improved
• 100% of Venous Ulcers with a Duration of One Year or Less Prior to VenoValve Surgery Have Fully Healed
• 89% of Venous Ulcers with a Duration of More Than One Year Priot to VenoValve Surgery have Fully Healed or Improved
• No Ulcer Recurrences
Venous Ulcer Healing
Setting New Standards for Venous Care
The VenoValve® is a first-in-class surgically implanted solution being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). Implanted into the femoral vein, the VenoValve is designed to act as a one-way valve to help restore proper blood flow up the leg, to return sufficient blood back to the heart. The VenoValve is currently being evaluated in the SAVVE® pivotal study.
Product
Highlights
-
FDA Breakthrough Device Designation
-
First device to receive IDE approval from the
FDA for the treatment of deep venous CVI
Product
Highlights
-
FDA Breakthrough Device Designation
-
First device to receive IDE approval from the
FDA for the treatment of deep venous CVI
Patient Experience From Pivotal SAVVE® Study
Interviews from the 50th Annual VEITH Symposium
Initial Topline Results from SAVVE® U.S. Pivotal Trial
Topline Efficacy Data
Clinical Improvement
Improvement in rVCSS
- 94%
94% of VenoValve Study Patients Showing Clinical Improvement at a Weighted Average of Eleven Months Post Surgery (rVCSS Improvement ≥ 1 point)
Clinical Meaningful Benefit
≥ 3 Point Improvement in rVCSS
- 72%
72% of the Study Patients Showing Clinical Meaningful Benefit from the VenoValve at a Weighted Average of 11 Months Post Surgery (Improvement in rVCSS of 3 or More Points)
Average rVCSS Improvement per Patient Showing Clinical Meaningful Benefit of 8.46 Points, More Than Two and a Half Times the Amount Needed to Show the VenoValve’s Clinically Meaningful Benefit
Compelling Device Related Safety Profile at 30 Days:*
0
No
Deaths
0
No Pulmonary
Embolisms
8%
Deep Vein Thrombosis
(DVT)**
*A higher than expected rate of procedure related pocket wound hematomas and wound infections was observed, with no negative impact on clinical improvement.
**Adjudication: 4 Mild; 2 Moderate. 5 of 6 DVT patients showing clinical improvement
Overview:
Preliminary 11 Month Efficacy Data
Preliminary Efficacy Data:
Clinical Meaningful Benefit Cohorts
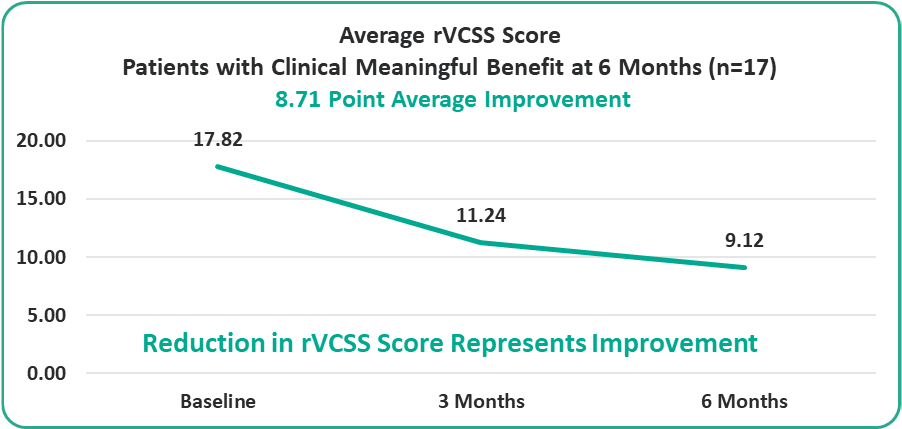
Preliminary Efficacy Data:
rVCSS Cohorts Subanalyses
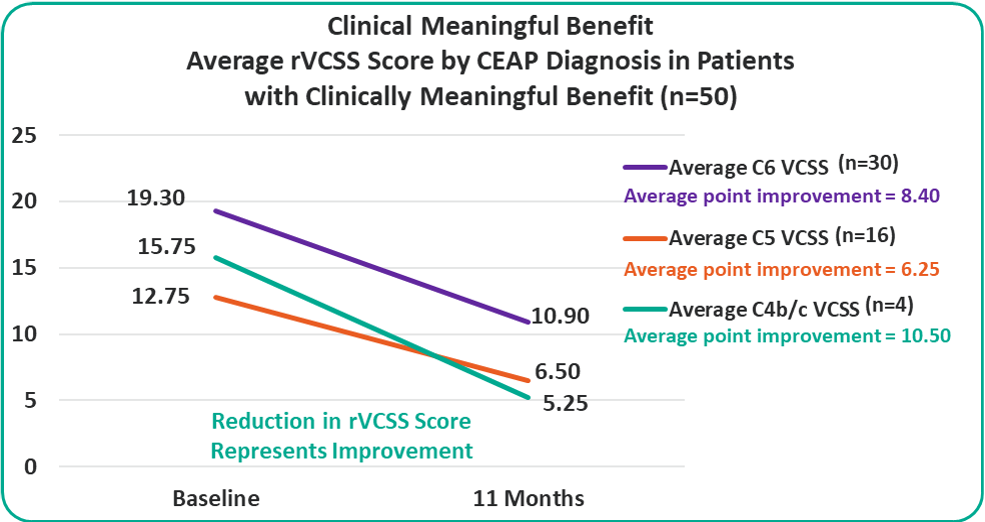
Patients with C4b/c, C5 and C6 are all benefiting from the VenoValve at 11 months compared to baseline.
Implantation Procedure
Chronic Venous Insufficiency
Scientific Publications
and Presentations