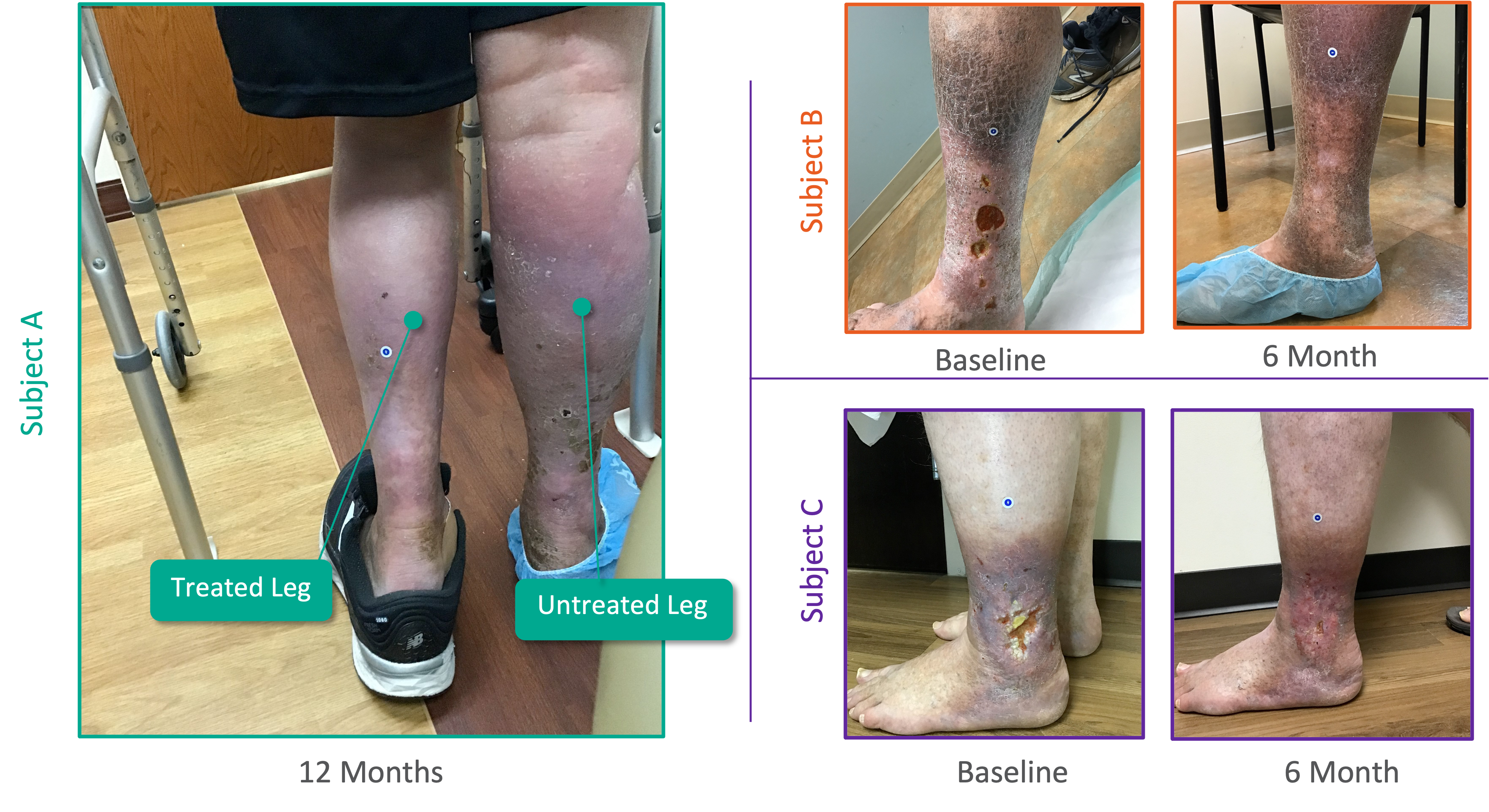
SAVVE® U.S. Pivotal Trial
Initial Positive 11-Month Topline Efficacy Data From SAVVE Trial*
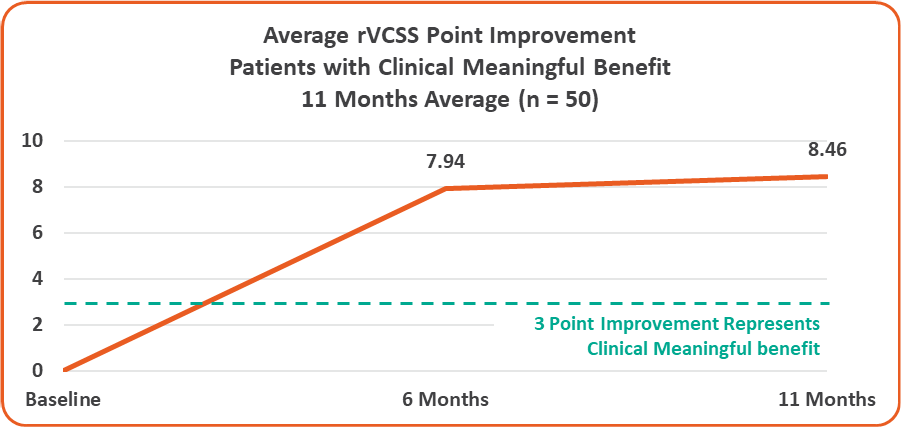
- Overall 8.46 Average Revised Venous Clinical Severity Score (rVCSS) Improvement Per Patient for Patients Showing Clinical Meaningful Benefit (rVCSS Improvement ≥ 3 Points) including:
- 9.29 Points for Patients at the Two-Year Milestone
- 8.08 Points for Patients at the One-Year Milestone
- 8.71 Points for Patients at the Six-Month Milestone
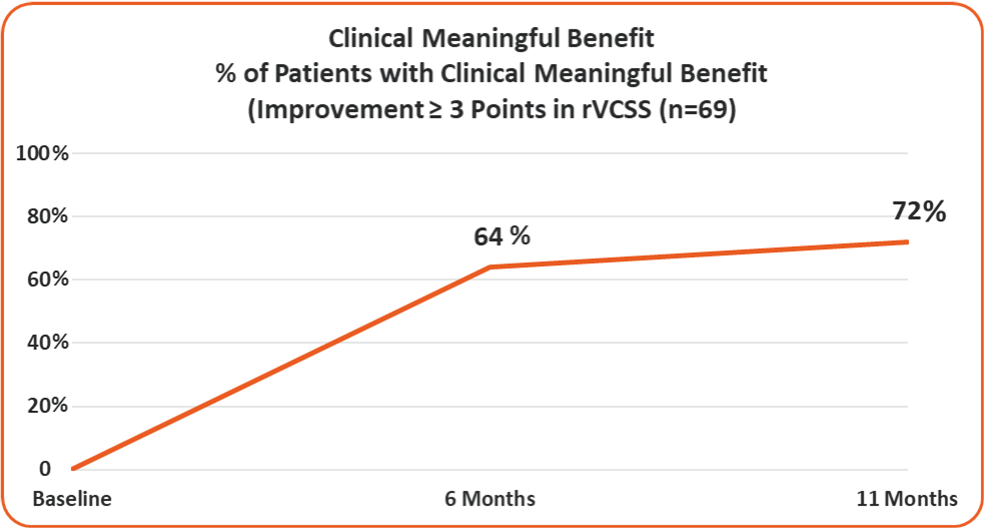
- 72% of the Study Patients Showing Clinical Meaningful Benefit from the VenoValve at a Weighted Average of 11 Months Post Surgery
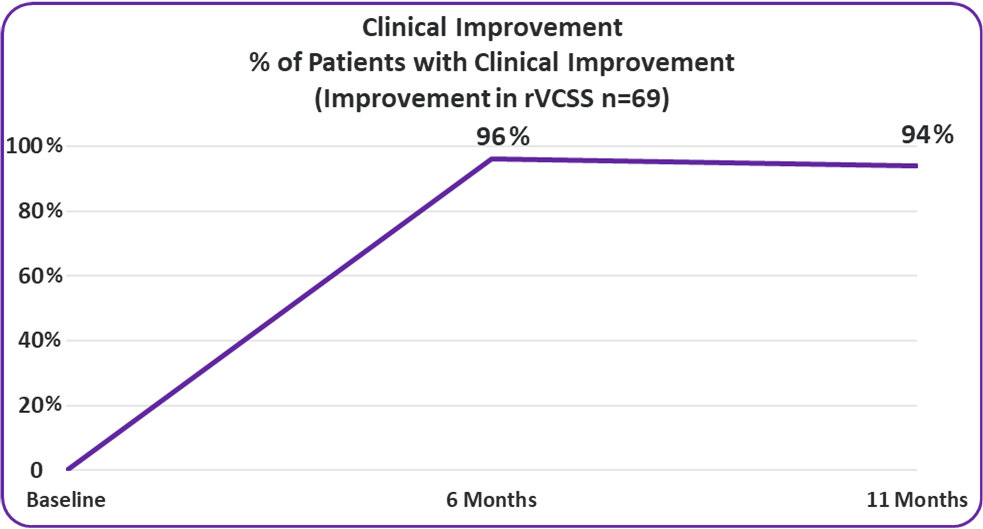
- 94% of VenoValve Study Patients Showing Clinical Improvement at a Weighted Average of Eleven Months Post Surgery (rVCSS Improvement ≥ 1 point)
* Data compared to baseline
Topline Efficacy Data
Clinical Improvement
Improvement in rVCSS
- 94%
94% of VenoValve Study Patients Showing Clinical Improvement at a Weighted Average of Eleven Months Post Surgery (rVCSS Improvement ≥ 1 point)
Clinical Meaningful Benefit
≥ 3 Point Improvement in rVCSS
- 72%
72% of the Study Patients Showing Clinical Meaningful Benefit from the VenoValve at a Weighted Average of 11 Months Post Surgery (Improvement in rVCSS of 3 or More Points)
Average rVCSS Improvement per Patient Showing Clinical Meaningful Benefit of 8.46 Points, More Than Two and a Half Times the Amount Needed to Show the VenoValve’s Clinically Meaningful Benefit
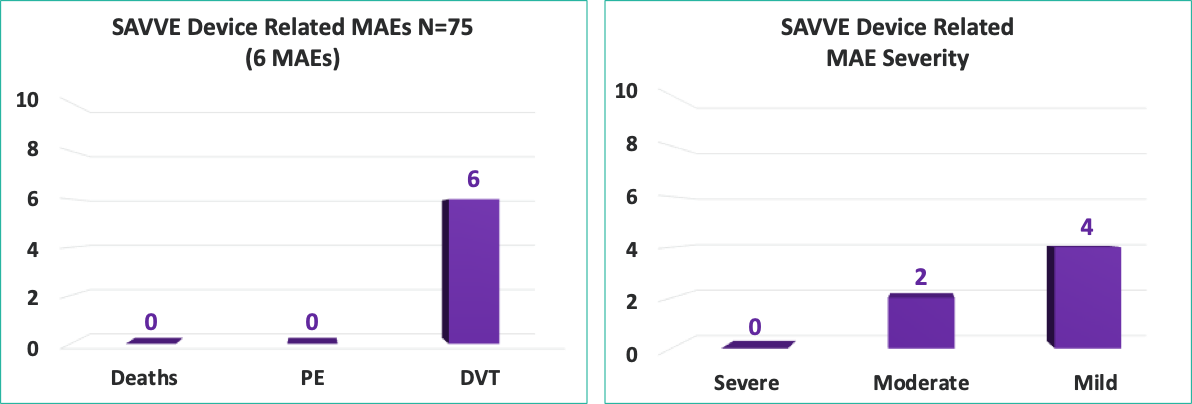
Compelling Device Related Safety Profile at 30 Days:*
0
No
Deaths
0
No Pulmonary
Embolisms
8%
Deep Vein Thrombosis
(DVT)**
*Higher than expected bleed rate was observed with no negative impact on clinical improvement
**Adjudication: 4 Mild; 2 Moderate. 5 of 6 DVT patients showing clinical improvement
Overview:
Preliminary 11 Month Efficacy Data
Preliminary Efficacy Data:
Clinical Meaningful Benefit Cohorts
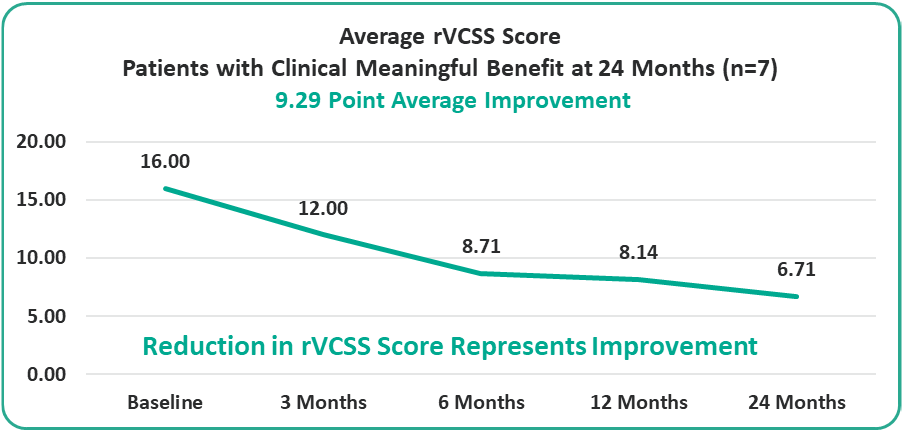
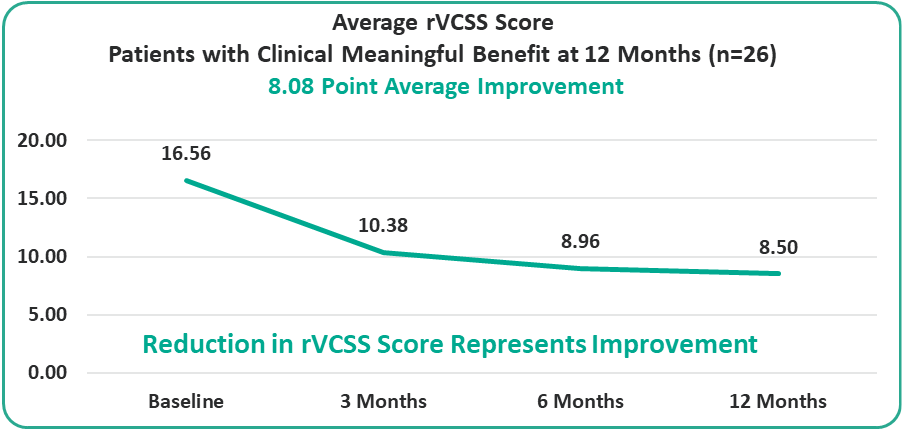
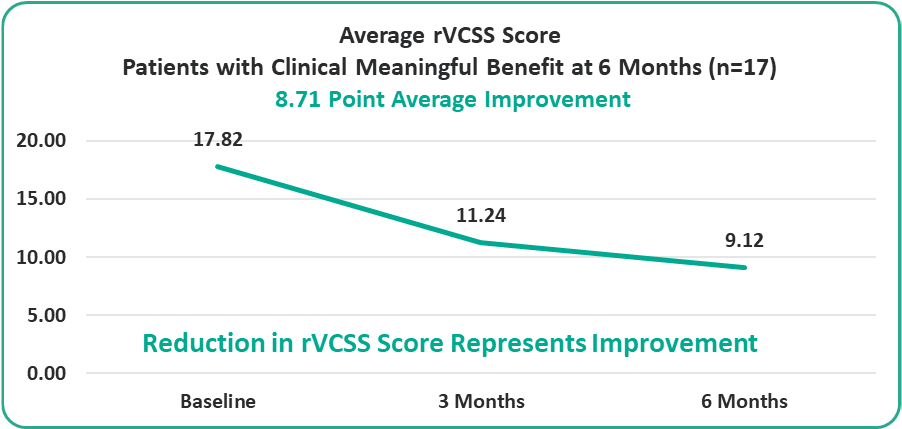
Preliminary Efficacy Data:
rVCSS Cohorts Subanalyses
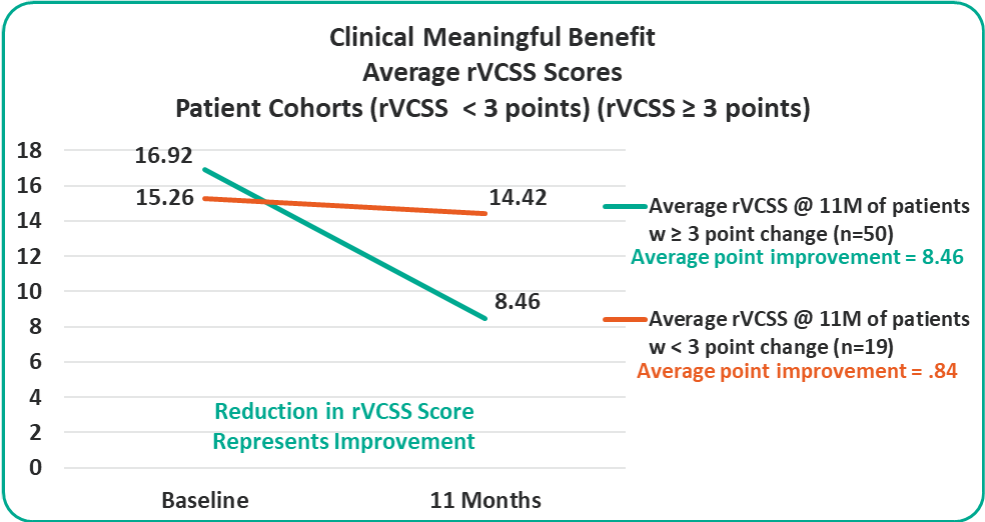
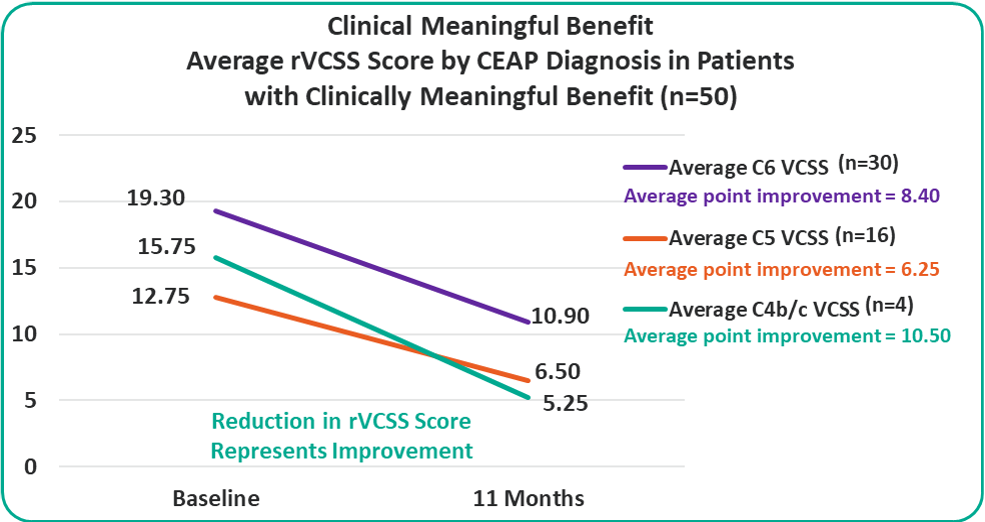
Patients with C4b/c, C5 and C6 are all benefiting from the VenoValve at 11 months compared to baseline.
Device Related MAEs (30 day)
Principal Investigators from Leading Surgical Centers Around the United States

Dr. Adriana Laser, MD
Albany Medical Center, Albany NY PI
Dr. Adriana Laser is fellowship trained and board certified in vascular surgery. She specializes in the operative and non-operative management of venous insufficiency, varicose veins, deep and superficial venous thrombosis, and lymphatic conditions.
Other areas of specialized interest are taking care of peripheral arterial disease, carotid artery disease, wound care, and arteriovenous access for hemodialysis patients.
Dr. Laser practices at Adriana Laser, MD Practice located at 1201 Nott St in Suite 202 in Schenectady, NY 12308 (Schenectady County).
Dr. Laser has 14 years of experience in vascular surgery.
Education
Adriana Laser, MD earned a degree of a Doctor of Medicine.
Licenses and Affiliations
Adriana Laser, MD has been registered with the National Provider Identifier database since June 07, 2007, and her NPI number is 1073716049.

Dr. Gregory Kasper, MD
Jobst Vascular Institute, Toledo, OH PI
President and Professor of Surgery Jobst Vascular Institute Promedica Healthcare System
Dr. Kasper has been practicing vascular surgery in Toledo for more than 15 years. In 2015, Dr. Kasper was named president and chief medical officer of Jobst Vascular Institute.
Dr. Kasper was born and raised in Toledo and returned after completing his medical training. Dr. Kasper and his wife, Christina, have three sons. Both Dr. and Mrs. Kasper are active in charitable work and enjoy travel and golfing.
Medical School
University of Cincinnati College of Medicine
Residency
Good Samaritan Hospital
Fellowship
Endovascular Fellowship, Arizona Heart Institute
Fellowship
Peripheral Vascular Surgery Fellowship, Good Samaritan Hospital
Providing leadership and strategic direction for the Promedica Healthcare System and the Jobst Vascular Institute. As Vice President of Medical Affairs I help align strategic goals with the expertise of our healthcare professionals to provide the highest quality cost efficient care.

Dr. Mikel Sadek, MD
NYU Langone Health – New York, NY PI
Associate Professor, Department of Surgery at NYU Grossman School of Medicine; Director, NYU Langone Vein Center; Associate Program Director, Vascular Surgery Residency and Fellowship
I was always fascinated with the human body, having grown up in a household of physicians. It was more than intellectual fascination—I knew that as a doctor I’d be able to lead a life of service. Moreover, I felt that if I could save one life in the course of my career, I would feel fulfilled.
Associate Professor, Department of Surgery at NYU Grossman School of Medicine; Director, NYU Langone Vein Center; Associate Program Director, Vascular Surgery Residency and Fellowship
I was always fascinated with the human body, having grown up in a household of physicians. It was more than intellectual fascination—I knew that as a doctor I’d be able to lead a life of service. Moreover, I felt that if I could save one life in the course of my career, I would feel fulfilled.
During medical school, I realized that surgeons are frequently the last line of defense between life and death. My lifelong profession was decided. I began my surgical training here, at NYU Langone, in 2004.
My interests drove me to specialize in vascular surgery, treating people with peripheral arterial disease, a condition affecting blood vessels. Part of the appeal was NYU Langone’s increasing use of minimally invasive endovascular techniques to repair blood vessels.
The use of minimally invasive techniques inspired me to research aortic aneurysms, participate in national meetings, and develop a more thorough understanding of vascular surgery. Since then, I’ve developed a particular interest in chronic venous insufficiency—weakened vein walls and valves—and in studying treatments that improve patients’ quality of life and advance the field of vascular surgery.
As a clinician, I want to create an environment in which I can have an open and clear dialogue with my patients. Treatment is often more successful when patients can participate in their own care and are educated by their physicians. Overall, I strive to provide the most effective and advanced combination of medical, endovascular, and open surgical techniques, as directed by the patient’s medical condition and needs.

Dr. Scott Berman, MD, MHA, RVT, FACS, DFSVS
Pima Heart and Vascular Tuscon, AZ PI
Hahnemann University School of Medicine
Dr. Berman received a bachelor’s degree in chemical engineering from Penn State University and a doctor of medicine degree from Hahnemann University. Following a general surgery residency in Virginia, he completed a vascular surgery fellowship at the University of Arizona. He opened his vascular surgery practice in Tucson in 1994 and has been the leader in southern Arizona vascular care introducing new technologies and treatment approaches including endovascular aneurysm repair for aortic aneurysms, carotid angioplasty and stenting, percutaneous peripheral vascular procedures and novel surgical techniques for hemodialysis access. He recently received a Master’s degree in Health Policy and Administration from Penn State University. He has authored over 50 peer-reviewed publications in the field of vascular disease along with 14 book chapters and a textbook on hemodialysis access. He is a member of regional, national and international societies devoted to the care of vascular disease patients. In recognition of his contributions, he was selected as a Distinguished Fellow for the Society of Vascular Surgery. He currently serves as the medical director for the Rocky Mountain Vascular Quality Initiative and is on the executive council of the Society for Vascular Surgery’s Patient Safety Organization.

Dr. David Dexter, MD
Sentara Hospital, Norfolk, VA PI
Dr. Dexter earned his bachelor's degree at Dartmouth College in Hanover, NH before enrolling in the State University of New York at Syracuse Upstate Medical University where he received his medical degree in 2004. He went on to University of Maryland Medical Center where he completed his general surgery training in 2010. During this time he also completed a one year fellowship in surgical research, publishing in several different subjects. In his last year he served as the administrative chief resident for the department of surgery. Dr. Dexter continued fellowship training in vascular and endovascular surgery at New York University in New York City. During his training he spent time abroad in Shanghai, China learning advanced aortic surgical techniques. As a published author, he has written and presented on arterial and venous disease at numerous medical conferences across the United States. As a vascular surgeon, Dr. Dexter is uniquely trained in the diagnosis and management of diseases affecting the vascular system, which includes arteries, veins and lymphatics, and specializes in endovascular surgery, the minimally invasive approach to vascular surgery. Dr. Dexter is certified by the American Board of Surgery. He is a member of the American College of Surgeons, the Peripheral Vascular Surgical Society, the Society of Vascular Surgery and the American Medical Association. Dr. Dexter practices with Sentara Vascular Specialists and sees patients at our Virginia Beach location.

Dr. Patrick Edward Muck, MD
TriHealth Heart Institute – Good Samaritan Hospital Cincinnati, OH PI
Professor of Surgery, Director, Wound Management Center
While his roots and educational background span the Tristate, Dr. Muck has gained national and international attention for his research and expertise in vascular surgery. A Northern Kentucky native and graduate of the University of Notre Dame, Dr. Muck chose to specialize in vascular surgery because of the immediate impact it can have on improving a patient's quality of life. Dr. Muck has shared his research and knowledge with peers at national and international meetings of many medical organizations. He also works with his colleagues at the TriHealth Heart Institute to host training courses to teach the newest vascular techniques to physicians from around the world. Dr. Muck specializes in minimally invasive treatment of all types of problems of the circulatory system, including: Aortic aneurysms, Carotid artery disease, Chronic venous insufficiency, Deep vein thrombosis, Peripheral vascular disease and Varicose veins. As director of the TriHealth Vascular Fellowship Program, Dr. Muck advances vascular care in the Tristate and nationally by training the next generation of vascular surgeons. He also has led numerous clinical trials at TriHealth, which offer the newest vascular treatments to patients.
SVS Document Oversight Committee member
Provide oversight and review of SVS documents and multispecialty consensus documents.

Dr. Cassius Iyad Ochoa Chaar MD, MS, RPVI
Yale School of Medicine – New Haven, CT PI
Associate Professor of Surgery, Division of Vascular Surgery
Dr. Cassius Iyad Ochoa Chaar, MD, MS, is an associate professor of surgery in the division of vascular surgery and endovascular therapy. He is seeing patients at the Yale Physicians Building. He is a board-certified in general surgery and vascular surgery, and is a registered vascular specialist in the interpretation of vascular laboratory studies. Dr. Chaar’s clinical interests include peripheral artery disease (PAD) focusing on patients with critical limb ischemia and limb salvage, varicose veins, aortic and visceral aneurysms, carotid disease, deep vein thrombosis, and dialysis vascular access. He completed his graduate and medical degrees at American University of Beirut, Lebanon. He spent his general surgery residency at Yale-New Haven Hospital, and subsequently finished a fellowship in vascular and endovascular surgery at University of Pittsburgh Medical Center.
Education & Training Fellow
University of Pittsburgh Medical Center (2011)
Chief Resident
Yale New Haven Hospital (2009)
Resident
Yale New Haven Hospital (2008)
Post-Doctoral research fellow
Cleveland Clinic Foundation (2004)
MD
American University of Beirut (2002)
MS
American University of Beirut (2000)

Dr. Michele Taubman, MD, FACS
Miami Vascular Specialists – Miami, FL PI
Miami Vascular Specialists/ Radiology Associates of South FL Endovascular and Vascular Surgery, Miami, FL
Dr. Michele Taubman was delighted to return to Miami following completion of her Vascular Surgery Fellowship at Emory University in Atlanta, GA, in 2015. She is a graduate of the University of Miami Medical School and General Surgery Residency at Jackson Memorial Hospital. Dr. Taubman’s training in complex endovascular and open vascular procedures both complements and elevates the services provided by Miami Vascular Specialists. Special interests include aortic aneurysms, peripheral arterial disease and limb salvage, dialysis access, varicose veins, and thoracic outlet syndrome. She also follows patients with carotid disease and offers surgical endarterectomy or transcarotid arterial revascularization (TCAR) when appropriate. She enjoys working closely with her patients and their families.
Dr. Taubman is fluent in Spanish and is Board Certified.

Dr. Rabih Chaer, MD
UPMC, Pittsburgh, PA PI
Dr. Rabih Chaer is a Professor of Surgery, Site chief of the Presbyterian campus of the division of Vascular Surgery at UPMC, and program director of the residency and fellowship programs at UPMC. Dr. Chaer joined the University of Pittsburgh and UPMC in 2006 after completing his vascular training at New York Presbyterian hospital of Columbia and Cornell. After joining UPMC, Dr. Chaer earned a master’s degree in clinical sciences at the University of Pittsburgh.
Dr. Chaer is a member of numerous professional and scientific societies, including the Association of Program Directors in Vascular Surgery, the American College of Surgeons, the Society for Vascular Surgery, the Society for Clinical Vascular Surgery, and the American Surgical Association. He is a distinguished fellow of the Society for Vascular Surgery and the American Venous Forum and is a past president of the Eastern Vascular Society. Author and co-author of over 200 peer reviewed publications. Dr. Chaer has also contributed to several clinical practice guidelines, book chapters and editorials and has given numerous national and international invited presentations on arterial and venous pathology. Dr. Chaer is a reviewer for the Journal of Vascular Surgery, Annals of Vascular Surgery, Annals of Surgery, and the Journal of Endovascular Therapy.
Dr. Chaer’s clinical research interests focus on AAA pathobiology and risk of rupture, and the genetic determinants of venous stasis ulcerations. He is also the co-PI of the Sunset PE trial, a randomized controlled trial comparing different modalities for PE catheter directed thrombolysis.
Dr. Chaer is interested in surgical education and wellness research, and currently serves on the research committee of the American Board of Surgery as well as the Video Based Assessment committee. Dr. Chaer is the past chair of the SVS Education council, is a current Councilor of the American Board of Surgery and a Director of the Vascular Surgery Board. He enjoys fitness training and the outdoors, including triathlons, and long distance running.

Dr. NavYash Gupta , MD
Cedars Sinai Medical Center,Los Angeles, CA PI
Dr. NavYash Gupta is an Associate Professor of Surgery at Cedars Sinai Medical Center and Medical Director of the Venous Program. He came to Cedars Sinai in July of 2017. Before coming to Cedars Sinai Dr. Gupta served as Chief of the Division of Vascular Surgery at NorthShore University HealthSystem in Evanston, Illinois from 2009 to 2017. Prior to that he was an Assistant Professor of Surgery at the University of Pittsburgh from 2000-2009. He completed his vascular training at the University of Chicago Medical Center in 1998.
Dr. Gupta is certified by the American Board of Surgery with a certificate of special qualifications in Vascular Surgery and is a Fellow of the American College of Surgeons. He is a member of numerous professional societies including the Society for Vascular Surgery, the Society for Clinical Vascular Surgery, the Western Surgical Association, the South Asian Association of Vascular Surgery and he was appointed SoCal VOICe representative for National Venous Research Advisory Council in January of 2020.
Dr. Gupta has been involved in clinical and translational research throughout his career resulting in multiple peer-reviewed publications and reports, reviews, book chapters and abstracts from national and international meetings. He has given numerous invited talks and has been a mentor for over 20 pre-medical students.
Among his other honors are the Patient Choice Award, Compassionate Doctor Recognition, Excellence in Teaching Award from the University of Chicago (2014) and being recognized as a Top Doctor by Los Angeles Magazine (2020, 2021 and 2022).
Dr Gupta has had a long-standing interest in hemodialysis access for patients with end stage renal disease as well as minimally invasive endovascular surgery for the treatment of venous disease, abdominal and thoracic aortic pathology, carotid artery occlusive disease and peripheral vascular disease.

Dr. Chelsea Dorsey, MD, RPVI
University of Chicago Medicine, Chicago, IL PI
Chelsea Dorsey, MD, is a board certified vascular surgeon with years of experience treating arterial and venous conditions. As the Director of the UCM Vein Clinic, she is dedicated to tackling venous disease with a multipronged approach including medical management, minimally invasive techniques, and surgical intervention. She will work with her patients to develop a plan of care that is both effective and patient-centered. A committed educator and mentor, Dr. Dorsey also regularly teaches medical students, residents and fellows about vascular disease management.
Specialties
Endovascular surgery, limb preservation, wound care, vein disorders.
Education
Undergraduate: BA, Rice University, Houston, Texas, 2006
Medical School: MD, University of Chicago Pritzker School of Medicine, Chicago, Illinois, 2010
Residency: Stanford University, Stanford, California, 2015
Board Certifications
Vascular Surgery – Board Certified (2016)

Dr. Claire Griffin, MD
University of Utah, Salt Lake City, UT PI
Dr. Claire Griffin grew up in England and moved to the United States in 1991. She received her Bachelor of Arts in Spanish from Florida State University before heading to medical school at the University of Florida. Her training continued at the University of Florida where she completed her residency in General Surgery. She went on to do a Vascular Surgery fellowship at Dartmouth Hitchcock Medical Center where her training included innovative endovascular approaches to the treatment of complex aortic disease, as well as open and endovascular techniques for limb salvage.
Dr. Griffin joined the University of Utah Department of Surgery and Division of Vascular Surgery with an interest in the entire spectrum of vascular pathology and a special interest in complex endovascular solutions to aortic disease.
Since arriving at the University of Utah, Dr. Griffin has pursued a Master's in Clinical Investigation with a particular focus on cost-effectiveness research. Additional research interests include heritability of aortic aneurysms and advancing shared decision making in the Vascular space. In 2017, Dr. Griffin, along with Dr. Jason Glotzbach in Cardiothoracic Surgery started the Aortic Disease Program which has subsequently gained institutional recognition as a Destination Care Program. Their work has significantly expanded the volume of thoracic aortic pathology treated at the University of Utah. Most recently, Dr. Griffin has become the Co-Director of the third-year surgery clerkship to expand upon her role as an educator.

Dr. Matthew Smeds, MD
Saint Louis University, Saint Louis, MO PI
Dr. Smeds is a tenured professor of surgery at Saint Louis University School of Medicine. He is the former chief of the division of vascular and endovascular surgery and program director for the vascular surgery training programs. He is an active clinician, educator, and researcher, with over 100 peer reviewed manuscripts and abstracts and has been involved in several clinical trials as institutional principal investigator. He is active in committees of the Society for Vascular Surgery, the Vascular Endovascular Surgery Society, and the American Venous Forum, and is the in-coming editor-in-chief of the Journal of Vascular Surgery Cases, Innovations, and Techniques.

Dr. Raghu Motaganahalli, MD
Indiana University School of Medicine, Indianapolis, IN PI
Dr. Raghu Motaganahalli, is a Professor of Surgery at the Indiana University School of Medicine and an attending Surgeon at the Indiana University Methodist Hospital. He is the Division Chief of Vascular Surgery and the Program director of vascular surgery training programs.
He completed his general and vascular surgery residency training at St Louis University School of Medicine , St Louis -Missouri . He is a distinguished fellow of the Society for Vascular Surgery, Fellow of The Royal College of Surgeons of Edinburgh, Fellow of the American College of Surgeons and an Associate member of the Academy of Master Surgeon Educators.
He serves as the President of the Midwestern Vascular Surgical Society (2021-22) besides serving as the Past-President of the Indiana State Chapter American College of Surgeons. He serves SVS, Midwestern Vascular Surgical Society , Association of Program Directors in Vascular Surgery, American Board of Surgery
He serves on the editorial board of Annals of Vascular Surgery, Vascular endovascular surgery, VSCORE . He is the principal investigator of over 15 clinical trials in vascular surgery at The Indiana University School of Medicine and currently serves as the US National Principal Investigator for DW-MRI Transcarotid revascularization. Dr. Motaganahalli, has over 100 peer-reviewed manuscripts, book chapters and abstracts. He routinely presents his work at several at national, international medical conferences.

Dr. Linda Harris, MD
University at Buffalo, Buffalo, NY PI
Dr. Linda Harris is a Professor of Surgery at the Jacobs School of Medicine and Biomedical Sciences, University at Buffalo. Clinically she primarily practices at Buffalo General Medical Center. She has been board certified in Vascular Surgery and General Surgery continuously since graduation. She is the program director of the residency and fellowship programs at UB, and served as the Division Chief for Vascular Surgery for over 2 decades. Dr. Harris joined the University of Buffalo in 1995 after completing her vascular training at UB.
Dr. Harris is a member and leader in numerous professional and scientific societies, including Past President of the Association of Program Directors in Vascular Surgery, Past President of the Eastern Vascular Society, and past Governor for the American College of Surgeons. She also serves on the Strategic Board of the Society for Vascular Surgery, and is a member of the Society for Clinical Vascular Surgery, the Society for University Surgeons, and the American Venous Forum. She has also organized and run the Womens Vascular Health Summit. She serves on the Intersocietal Accreditation Commission, and has served on the Residency Review Committee of the Accreditation Council for Graduate Medical Education. She is a distinguished fellow of the Society for Vascular Surgery. She has authored and co-authored over 100 peer reviewed publications, and 20 book chapters, including serving as editor for 2 vascular textbooks, and associate editor for another. She has also given numerous national and international invited presentations on a wide array of vascular diseases. Dr. Harris sits on the editorial board for the Journal of Vascular Surgery, the Journal of Vascular Surgery, Venous & Lymphatic, and also reviews for the European Journal of Vascular and Endovascular Surgery, and Annals of Vascular Surgery, as well as several other journals.
Dr. Harris’s clinical research interests focus on peripheral arterial disease, arteriovenous dialysis access, aortic aneurysms, as well as treatment of deep vein thrombosis. She also has a significant interest in surgical education.
She currently serves as a senior examiner for the American Board of Surgery for the vascular surgical examination, and has participated in the committee on Maintenance of Certification for the Vascular Board, writing questions for the exam for several years. Dr. Harris is the current chair of the SVS Education council.

Dr. Marc Passman, MD
University of Alabama at Birmingham, Birmingham, AL PI
Dr. Passman is currently on faculty at University of Alabama at Birmingham (UAB) in the Division of Vascular Surgery & Endovascular Therapy, Department of Surgery as a Professor of Surgery. He is an American Board of Surgery diplomate in vascular surgery and practices the full spectrum of arterial and venous surgery. He is also the Director of the UAB Vein Program which provides inpatient and outpatient care to patients with a range of venous problems. Dr. Passman earned his AB degree in biochemistry and government, graduating magna cum laude from Bowdoin College in Brunswick, Maine. In 1991, he received his M.D. degree from the University of Vermont’s School of Medicine in Burlington and then completed an internship and residency in general surgery at the Oregon Health Sciences University in Portland (1991-1997). He completed a fellowship in vascular surgery at the University of North Carolina at Chapel Hill (1999). He was previously on faculty at Vanderbilt University Medical Center, before joining the UAB faculty in 2006. He has authored numerous journal articles and book chapters and is on the editorial board for the Journal of Vascular Surgery, and Annals of Vascular Surgery. He is also involved in several national medical societies, holding leadership positions in the Peripheral Vascular Society, and the American Venous Forum, and the Society for Vascular Surgery. He is past president of the American Venous Forum, and the Peripheral Vascular Surgery Society.

Dr. Karem Harth, MD, MHS, RPVI
University Hospitals Cleveland Medical Center, Cleveland, OH PI
Karem C. Harth, MD, MHS, RPVI, is a board-certified vascular surgeon and registered physician in vascular interpretation. She serves as Director of the Center for Comprehensive Venous Care and Co-Medical Director of Vascular Laboratories at University Hospitals Harrington Heart & Vascular Institute. In addition, she is an Assistant Professor of Surgery at Case Western Reserve University School of Medicine.
Dr. Harth earned her medical degree from the Stony Brook School of Medicine in New York. She also completed a Master of Health Science at Johns Hopkins Bloomberg School of Public Health. Dr. Harth then completed a general surgery residency and vascular surgery fellowship at University Hospitals Cleveland Medical Center.
Dr. Harth is an active member of multiple national professional organizations, including the Society for Vascular Surgery, American Venous Forum, Society for Vascular Ultrasound, American College of Surgeons and Society for Clinical Vascular Surgery (SCVS). She has given multiple lectures at regional, national and international society meetings.
Dr. Harth has been a contributing author on over 100 articles and abstracts in peer-reviewed journals and major societal meetings. She also serves as a manuscript reviewer for such highly regarded publications as Vascular and Endovascular Surgery, Journal of Vascular Surgery, Journal of Vascular Medicine, and Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Dr. Harth treats a wide range of vascular diseases and is especially interested in the treatment of venous disease. She has earned research support related to chronic venous thrombosis, ventral hernia repair and vascular access techniques. She is a recipient of the Faculty Teaching Award in the Division of Vascular Surgery and Endovascular Therapies at UH Cleveland Medical Center and has been named multiple times as a Top Doctor by Cleveland Magazine.

Dr. Vincent DiGiovanni, MD
Lankenau Medical Center, Wynnewood, PA PI
Dr. Digiovanni is a Vascular Surgeon and has been practicing medicine for over 17 years. He is highly rated in 15 conditions, with his top areas of expertise being Venous Insufficiency, Stasis Dermatitis and Ulcers, Peripheral Artery Disease, Carotid Artery Disease, and Carotid Artery Surgery.

Dr. Kathleen Ozsvath, MD
St. Peter's Vascular Associates, Troy, NY PI
Dr. Kathleen Ozsvath, MD specialized in vascular surgery with over 30 years of experience in medicine. Dr. Ozsvath has extensive experience in Carotid Artery Disease or Carotid Endarterectomy, Peripheral Vascular Disease, and Aortic Aneurysm or Dissection.
Study Design:
Prospective, Non-Blinded, Single Arm, Multi-Center Study

Primary
Safety Endpoint
30 DAYS
Efficacy Endpoints
180 DAYS
Inclusion:
Failure of at Least 3 Months Standard Care
Axial Reflux >1 Second
Valvular Incompetence (Primary or Secondary)
CEAP Score: C4b, C5, C6
Ability to Ambulate Without Assistance
ABI >.61
BMI <40
Exclusion:
Hypercoagulable Condition
Acute Deep Venous Thrombosis or Pulmonary Embolism
Lymphedema
Superficial Reflux
Iliac/IVC Obstruction or Poor Central Venous Flow
Uncontrolled Diabetes Mellitus
Sepsis
Patient Characteristics
Demographics:
Age: 63 [38 – 83]
Mean BMI: 32.3
Male: 81.3% (N=61)
Race
White: 86.7% (N=65)
African American: 12.0% (N=9)
Other: 1.3% (N=1)
CEAP Classification:
70.5% (N= 31) of Patients With C6 Disease Had Ulceration for More Than One Year
C4b: 5/75 (6.7%)
C4c: 5/75 (6.7%)
C5: 21/75 (28.0%)
C6: 44/75 (58.7%)
Comorbidities:
Diabetes: 32.0% (N=24)
Peripheral Artery Disease: 6.7% (N=5)
Chronic Venous Insufficiency
Publications