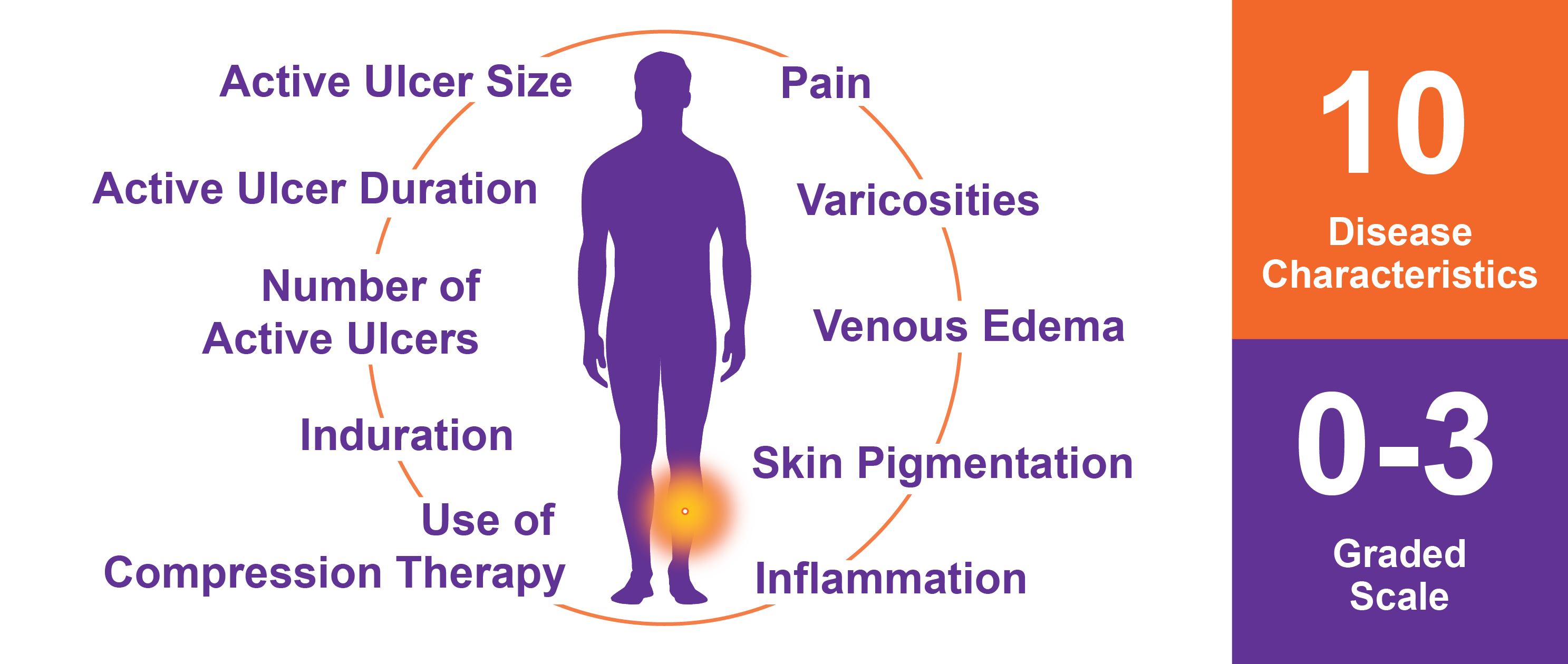
Venous Clinical Severity Score (rVCSS)
The revised Venous Clinical Severity Score (rVCSS) is an objective grading system used by vascular specialists throughout the world to measure the severity of venous diseases, such as Chronic Venous Insufficiency (CVI), and to report clinical outcomes and responses to treatments for venous diseases. The revised Venous Clinical Severity Score (rVCSS) consists of patient and physician reported outcomes for ten venous disease characteristics that are graded from 0 to 3.
For the non-practitioner, as used in the VenoValve® pivotal trial, a comparison of a patient’s Venous Clinical Severity Score at baseline (Pre-VenoValve) to the patient’s rVCSS assessment at subsequent times (Post-VenoValve) provides an answer to the question – “Has the patient’s CVI improved?”
The rVCSS therapeutic goal for the VenoValve pivotal trial is a 3 point rVCSS improvement, when comparing baseline rVCSS assessments to subsequent assessments at 90 days, 180 days and 1 year post VenoValve implantation. A 3 point improvement in rVCSS for patients with severe CVI would be evidence of the VenoValve’s clinically meaningful benefit.
rVCSS: Assess the Severity of Chronic Venous Disease
3 Point Improvement rVCSS = Evidence of Clinically Meaningful Benefit
The revised Venous Clinical Severity Score (rVCSS) is one of several clinical endpoints that will be evaluated by the FDA to determine whether the VenoValve® provides clinical meaningful benefit for patients with severe CVI.
Measuring rVCSS Endpoint in VenoValve® U.S. Pivotal Trial
Baseline rVCSS is evaluated pre-surgery for each patient and is compared to rVCSS evaluations at:

The FDA will evaluate 16 different safety and efficacy endpoints throughout the VenoValve® pivotal trial and will base its approval determination on the totality of the evidence. The FDA has requested one-year data on all patients as a prerequisite to filing for FDA approval.
Venous Disease
VenoValve®
SAVVE® Clinical Study
The VenoValve® and enVVe® are investigational medical devices currently in development. Neither device is approved or cleared for any indication in any market. The VenoValve® is only available for use in the United States in pre-market clinical studies.