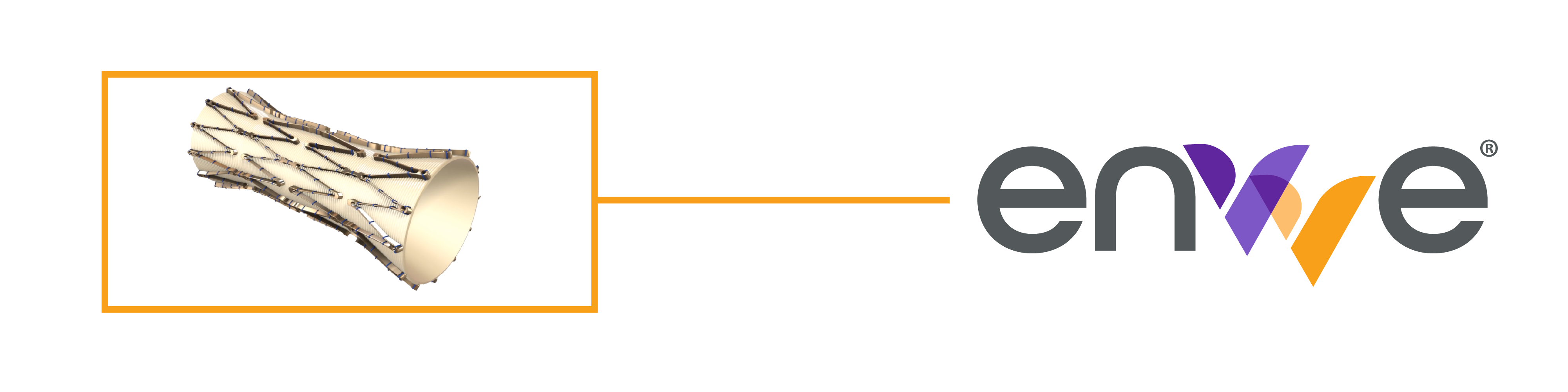
Next-Generation, Non-Surgical Replacement Venous Valve for Treatment of Chronic Venous Insufficiency (CVI) in the Deep Veins of the Leg
enVVe® is our next-generation, non-surgical, transcatheter-based replacement venous valve, being developed for the treatment of CVI of the deep veins of the leg. Building on our extensive experience in CVI with the development of the VenoValve®, enVVe® potentially expands our total addressable market to include people living with less severe CVI and people with co-morbidities or for whom an open surgical procedure may pose too much risk.
Product Highlights
-
Minimally invasive procedure requiring no general anesthesia and no overnight hospital stay
-
Procedure to be performed in an Angio Suite as opposed to an operating room
-
Self-expanding frame made from a specially formulated biocompatible nickel and titanium alloy
-
Frame geometry that accommodates the natural dilation and contraction of the vein, and provides a proper fit across a broad range of vein sizes
-
Mono-cusp leaflet that is laser cut from porcine pericardium tissue
-
Delivery profile of only 13 Fr (4.3 mm) when crimped, giving it the smallest profile of any replacement valve currently in use for the cardiovascular system
-
Delivery via an over-the-wire, coaxial, single-stage pull system for ease of use

Minimally Invasive Implantation Procedure
Chronic Venous Insufficiency
VenoValve®
SAVVE® Clinical Study
Scientific Publications
and Presentations
The VenoValve® and enVVe® are investigational medical devices currently in development. Neither device is approved or cleared for any indication in any market. The VenoValve® is only available for use in the United States in pre-market clinical studies.