
First-In-Human (FIH) Study
Transcatheter Anti-Reflux, Venous Valve Endoprosthesis (TAVVE)
What is the TAVVE® Study?
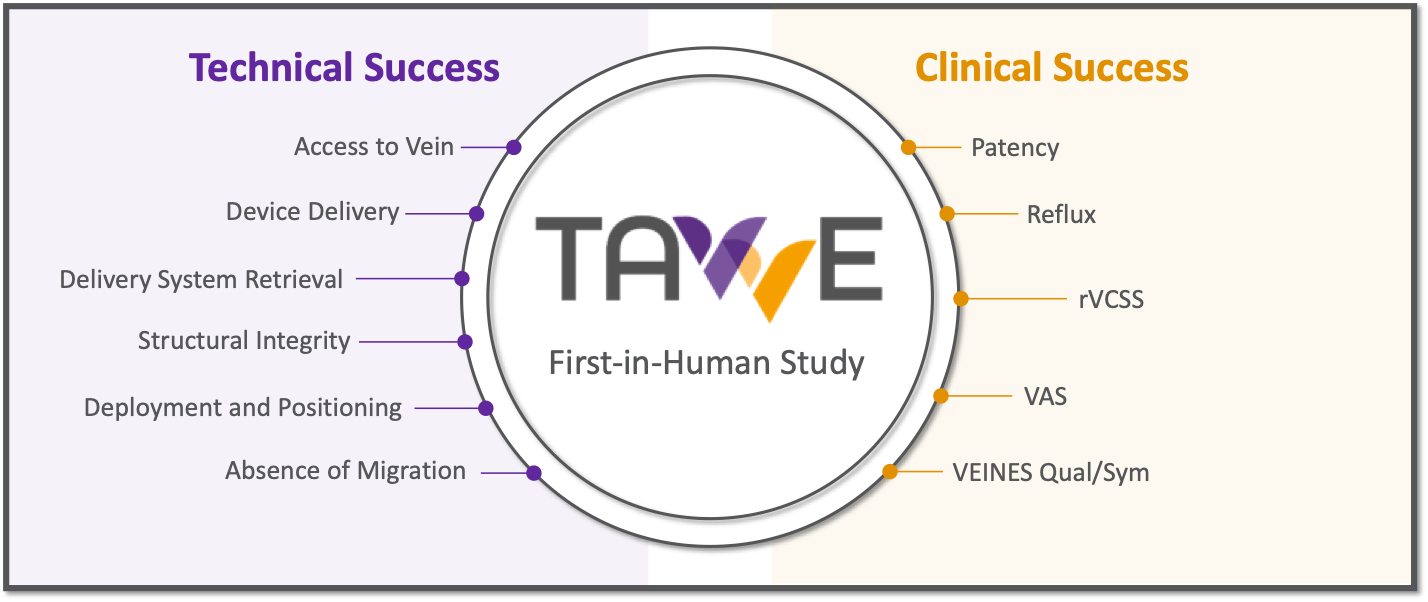
The initial phase of the TAVVE-FIH study will seek to enroll 3 to 5 patients across multiple sites. Several parameters will be evaluated over the course of the study including safety and technical success of the enVVe® venous valve delivery system, and the safety and clinical performance of the enVVe® venous valve.
Chronic Venous Insufficiency
enVVe®
Publications
The VenoValve® and enVVe® are investigational medical devices currently in development. Neither device is approved or cleared for any indication in any market. The VenoValve® is only available for use in the United States in pre-market clinical studies.


